Pfizer announces positive results from trial of COVID-19 vaccine in children 5 to 11 years
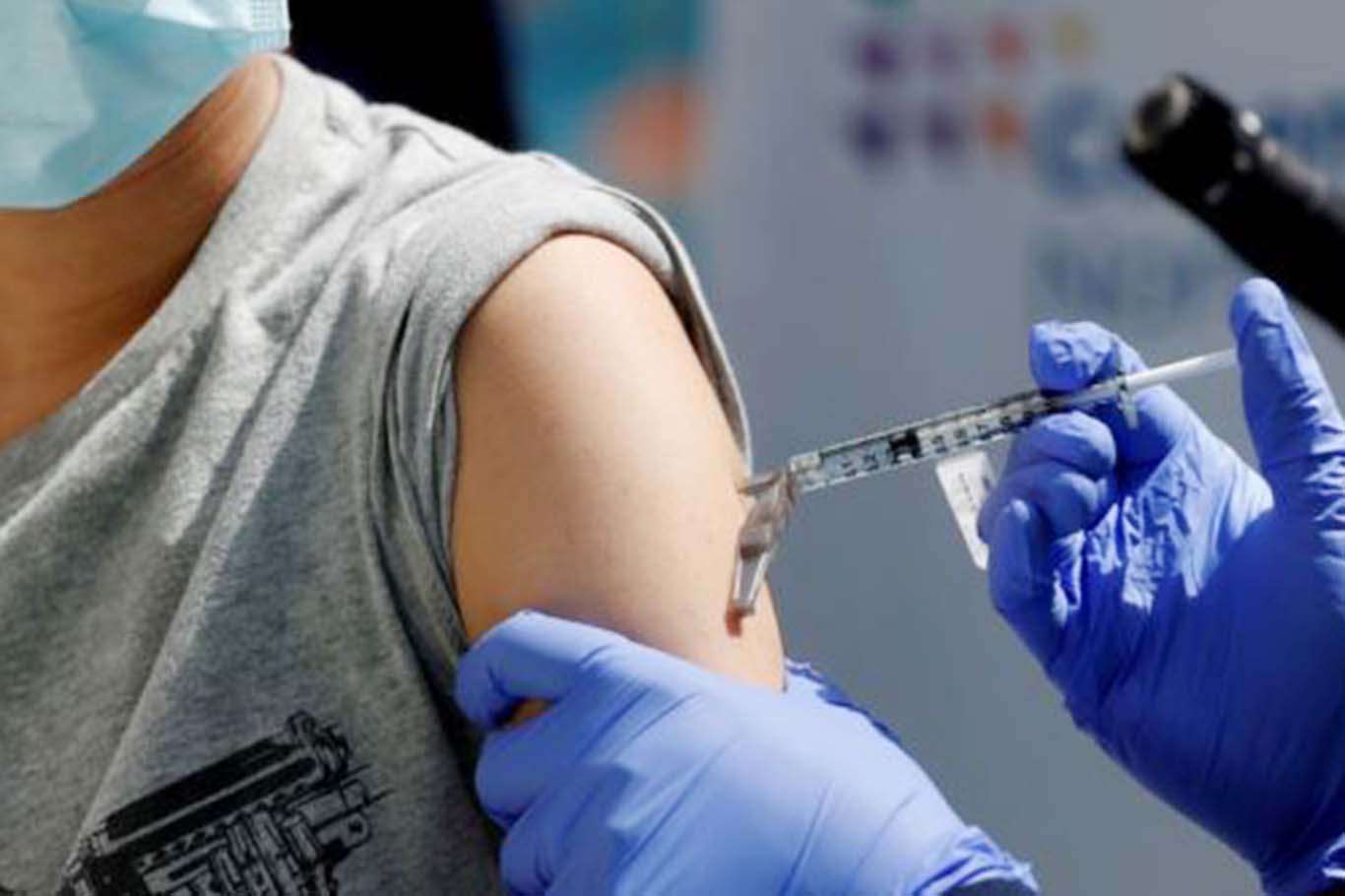

“In participants 5 to 11 years of age, the vaccine was safe, well-tolerated and showed robust neutralizing antibody responses,” the company said.
Pfizer announced on Monday results from a Phase 2/3 trial showing a favorable safety profile and robust neutralizing antibody responses in children 5 to 11 years of age using a two-dose regimen of 10 µg administered 21 days apart, a smaller dose than the 30-µg dose used for people 12 and older.
“The antibody responses in the participants given 10 µg doses were comparable to those recorded in a previous Pfizer-BioNTech study in people 16 to 25 years of age immunized with 30 µg doses. The 10 µg dose was carefully selected as the preferred dose for safety, tolerability, and immunogenicity in children 5 to 11 years of age. These are the first results from a pivotal trial of a COVID-19 vaccine in this age group,” the company said.
“Over the past nine months, hundreds of millions of people ages 12 and older from around the world have received our COVID-19 vaccine. We are eager to extend the protection afforded by the vaccine to this younger population, subject to regulatory authorization, especially as we track the spread of the Delta variant and the substantial threat it poses to children,” said Albert Bourla, Chairman and Chief Executive Officer, Pfizer. “Since July, pediatric cases of COVID-19 have risen by about 240 percent in the U.S. – underscoring the public health need for vaccination. These trial results provide a strong foundation for seeking authorization of our vaccine for children 5 to 11 years old, and we plan to submit them to the FDA and other regulators with urgency.”
“We are pleased to be able to submit data to regulatory authorities for this group of school-aged children before the start of the winter season,” said Dr. Ugur Sahin, CEO, and co-founder of BioNTech. “The safety profile and immunogenicity data in children aged 5 to 11 years vaccinated at a lower dose are consistent with those we have observed with our vaccine in other older populations at a higher dose.” (ILKHA)
LEGAL WARNING: All rights of the published news, photos and videos are reserved by İlke Haber Ajansı Basın Yayın San. Trade A.Ş. Under no circumstances can all or part of the news, photos and videos be used without a written contract or subscription.
President Recep Tayyip Erdoğan inaugurated the Medistate Çekmeköy Hospital on Friday, framing the event as a testament to Türkiye’s advanced and inclusive healthcare model, which he described as "one of the most beautiful examples of our human-centered politics."
Amid persistent challenges, the past year has delivered historic victories in the global fight against infectious diseases, marking a turning point in several long-standing public health battles.
A new pilot study suggests that neurofeedback-based digital training may significantly enhance brain function in children diagnosed with dyslexia, offering fresh hope for non-invasive, long-term interventions targeting reading-related neurological challenges.
Influenza cases are rising sharply across Europe, placing growing pressure on hospitals and health systems in many countries, the World Health Organization (WHO) has warned.